
Introduction:
Prognosis for adult B-cell Acute Lymphoblastic Leukaemia (B-ALL) is poor and there is currently no licensed CD19 Chimeric Antigen Receptor (CAR) therapeutic. We developed a novel CD19 CAR (CAT-41BBz CAR) with a fast off-rate, designed to result in more physiological T-cell activation, reduce toxicity and improve engraftment. We describe updated data from the Phase I ALLCAR19 (NCT02935257) study of AUTO1 in relapsed/refractory adult B-ALL.
Methods:
Manufacturing: AUTO1 utilises non-mobilised autologous leukapheresate. The first 6 products were generated using a standard dynabead/WAVE bioreactor process and subsequent products using a semi-automated closed process.
Study design: Patients aged >16y underwent lymphodepletion with fludarabine (30mg/m2 x3) and cyclophosphamide (60mg/kg x1) followed by split dose CAR T-cell infusion (Day 0: if ≥20% Bone Marrow (BM) blasts, infuse 10 x 106 CAR T-cells; if <20% BM blasts, 100 x 106 CAR T-cells. At Day+9: if no grade 3-5 Cytokine Release Syndrome (CRS)/immune effector cell associated neurotoxicity syndrome (ICANS), infuse Dose 2, to a total dose of 410 x106 CAR T-cells). Study endpoints include feasibility of manufacture, grade 3-5 toxicity and remission rates at 1 and 3 months.
Results:
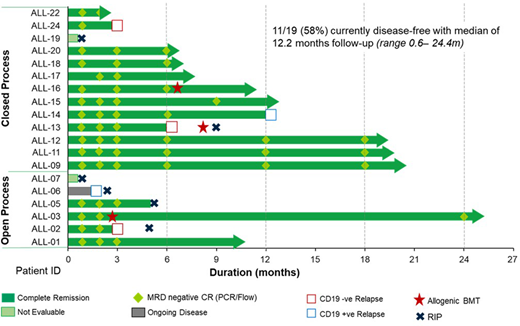
As of 13 May 2020, 24 patients have been leukapheresed, 23 products manufactured and 19 patients received at least 1 dose of AUTO1. The median age was 43y (range 18-62), 26% had prior blinatumomab, 47% had prior inotuzumab ozogamicin and 63% had prior hematopoietic stem cell transplantation (HSCT). At the time of pre-conditioning, 42% had ≥50% BM blasts.
No patients experienced ≥Grade 3 CRS (Lee criteria), 3/19 (16%) experienced Grade 3 ICANS that swiftly resolved with steroids. Of 19 infused patients, 16/19 (84%) achieved Minimal Residual Disease (MRD) negative complete response (CR). Currently 6 patients have died, none related to AUTO1. 11/19 (58%) patients remain on study and continue in MRD negative remission at a median follow up of 12.2 months (range 0.6-24.4m). To date, only 2 patients underwent HSCT whilst in remission. For all treated patients, the event-free survival (EFS) at 6 months was 62% and 76% for those whose products were manufactured using the closed process. Patients exhibited robust CAR expansion (mean peak CAR T levels 716,769 copies/µg DNA).
Conclusions:
AUTO1 has a tolerable safety profile in adult patients with r/r B-ALL despite high disease burden. Early data shows high remission rates with 84% achieving MRD negative CR. This preliminary data supports the further development of AUTO1 as a standalone treatment in patients with r/r B-ALL. Data from additional patients and longer follow up will be presented. Furthermore, data from extension cohorts of patients with low- and high- grade B-cell Non-Hodgkin's lymphoma and chronic lymphocytic leukaemia will be presented.
Roddie:Celgene: Honoraria; Gilead: Honoraria; Novartis: Honoraria. O'Reilly:Gilead: Honoraria; Novartis: Honoraria, Other: Travel support. Hartley:ADC Therapeutics: Consultancy, Current equity holder in publicly-traded company, Research Funding. Linch:Autolus: Consultancy. Pule:UCLB: Patents & Royalties; Mana Therapeutics: Other: entitled to share of revenue from patents filed by UCL; Autolus: Current Employment, Other: owns stock in and receives royalties, Patents & Royalties. Peggs:Autolus: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal